Research
Our current research has three main themes.
Mechanistic understanding of sustainable aqueous batteries
Aqueous batteries emerge as attractive alternatives for large-scale energy storage by offering several advantages, such as non-flammability, cost-effectiveness, and environmental benignity. However, a notable drawback of aqueous electrolytes is the limited battery cell voltage. The narrow electrochemical stability window of water (1.23 V) triggers hydrogen evolution reaction and oxygen evolution reaction during battery overcharging. Unlike some organic electrolytes, water does not form electrode passivation layers and rapid battery failure is often observed.
Our research team conducts physicochemical and electrochemical testing to elucidate the working mechanisms of model electrodes, such as layered oxide (e.g., vanadium pentoxide, V2O5) and layered sulfide (e.g., titanium disulfide, TiS2), in sustainable aqueous alkali-ion batteries with dilute electrolytes [1-3].
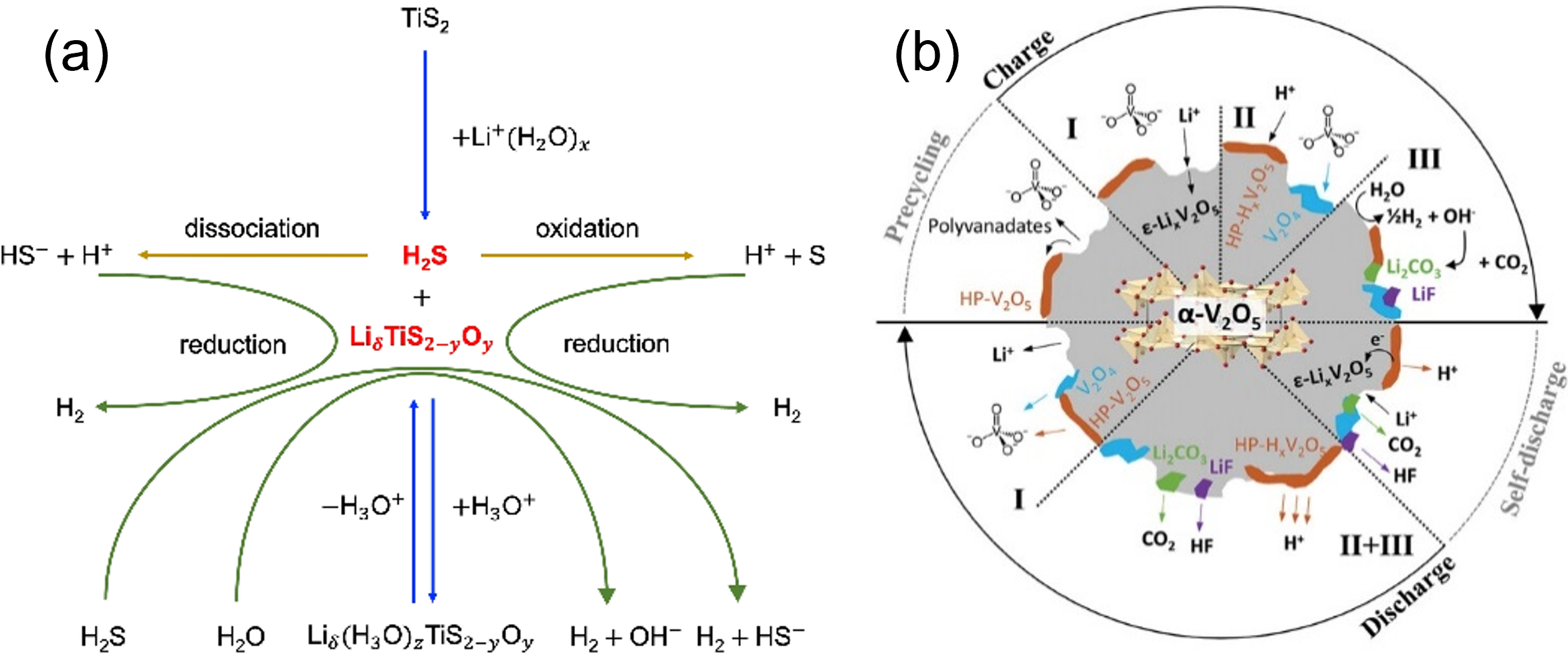
Collaborators: Chao Zhang, William Brant (Uppsala University), and MAX IV Laboratory (Sweden).
References:
- Zhang et al. Reversible Hydration Enabling High-Rate Aqueous Li-Ion Batteries. ACS Energy Letter. 2024, 9 (3), 959–966.
- Hou et al. Interfacial Chemistry in Aqueous Lithium-Ion Batteries: a Case Study of V2O5 in Dilute Aqueous Electrolytes. Small 2023, 2308577.
- Zhang et al. Reactivity of TiS2 Anode Towards Electrolytes in Aqueous Li-Ion Batteries. Batter. Supercaps 2022, 5 (12), e202200336.
Development and application of advanced operando characterizations
To investigate the battery performance degradation, researchers typically need to stop the cell, extract and prepare electrodes and/or electrolytes for ex situ analysis. However, such conditions typically distort the actual working environment of the sample under study. In contrast, operando techniques allow for continuous analysis without interrupting the cell, providing a more authentic understanding of transient changes occurring inside functioning batteries in real-time.
We possess extensive experience in developing operando techniques, including online electrochemical mass spectrometry (OEMS, for gas evolution), electrochemical quartz-crystal microbalance with dissipation monitoring (EQCM-D, for solid deposition), online acoustic emission testing (AE, for mechanical degradation), and operando pH monitoring (for acid-base reaction) [1-3].
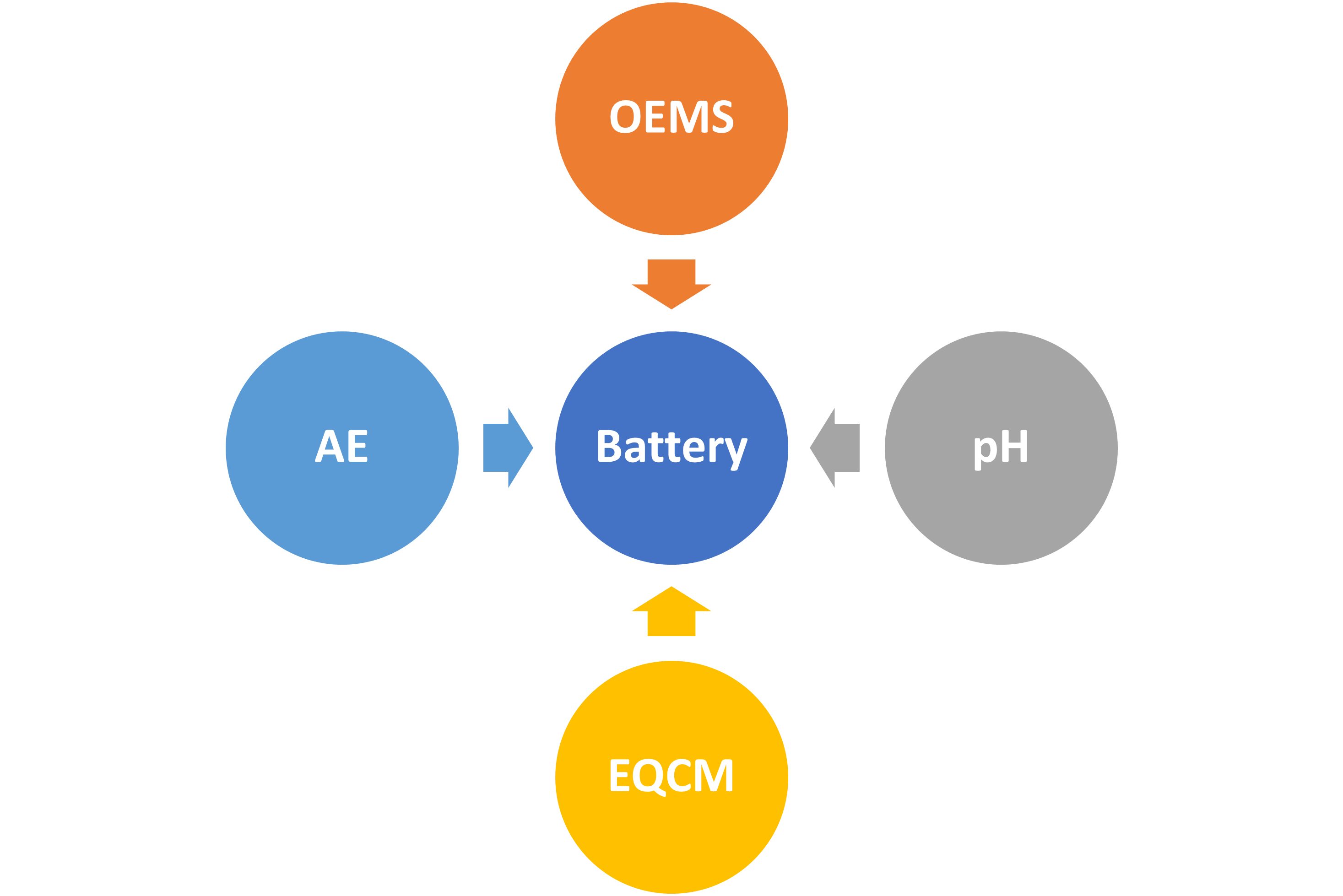
Collaborators: Annika Ahlberg Tidblad (Volvo Cars), Jean-Marie Tarascon (Collège de France), and Qi Zhao (HK PolyU).
References:
- Li et al. Decoupling the Roles of Ni and Co in Anionic Redox Activity of Li-Rich NMC Cathodes. Nat. Mater. 2023, 22 (11), 1370–1379.
- Zhang et al. Elucidating the Humidity-Induced Degradation of Ni-Rich Layered Cathodes for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14 (11), 13240–13249.
- Zhang et al. Unraveling Gas Evolution in Sodium Batteries by Online Electrochemical Mass Spectrometry. Energy Storage Mater. 2021, 42, 12–21.
High-throughput robotic screening of aqueous electrolytes
The integration of data-driven experimentation represents a significant advancement in battery research, substantially expediting progress by closing the experimentation–analysis loop. In contrast to the traditional manual screening of electrodes and electrolytes, automated processes hold the promise of improving both testing efficiency and reproducibility.
At UU, we have pioneered the development of ODACell, an automated platform for electrolyte formulation preparation and coin cell assembly, equipped with three robotic arms and a liquid-handling robot [1]. Our investigations demonstrate that cells assembled by the robots exhibit exceptional reproducibility and consistency. Presently, we are implementing Bayesian optimization to systematically screen additives for high-performance and low-cost aqueous Li-ion batteries.
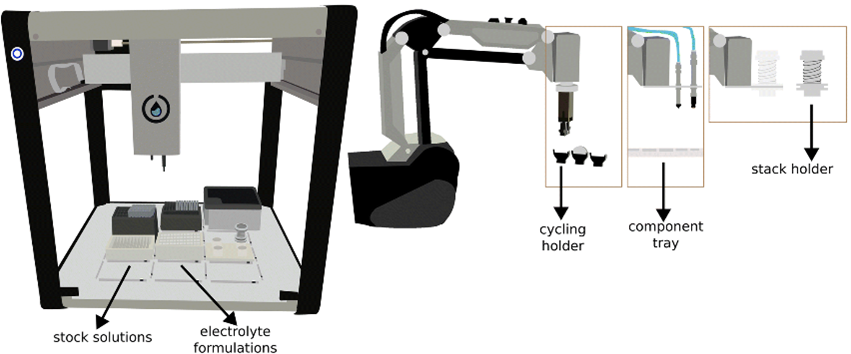
Collaborators: Erik Berg and Jens Sjölund (Uppsala University).
References:
- Yik et al. Automated Electrolyte Formulation and Coin Cell Assembly for High-Throughput Lithium-Ion Battery Research. Digit. Discov. 2023, 2 (3), 799–808.
